Estudio del estado de aglomeración de partículas mediante RMN de campo bajo
Agglomeration State of Particles
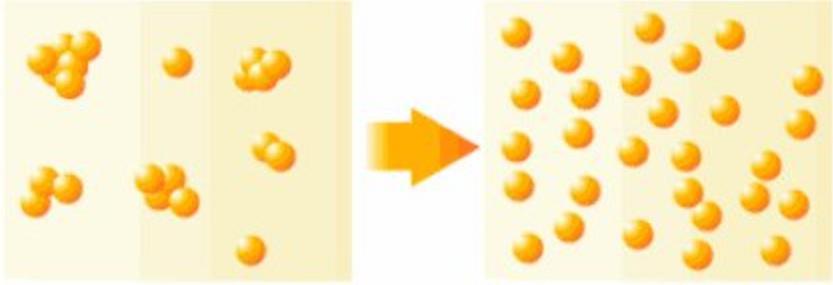
The agglomeration of particles can be divided into three states according to their mechanism of action:
Aggregate: Refers to the primary particles connected by faces, and their surface area is much smaller than the sum of their individual particles, and it is very difficult to re-disperse in this state.
Agglomerate: refers to the attachment of primary particle clusters or small particles to large particles connected by points and corners, y su superficie total es mayor que la de los aglomerados, pero más pequeño que la suma de las partículas individuales, y la redispersión es relativamente fácil. Los aglomerados y aglomerados también se denominan partículas secundarias..
Flocculation: refers to the looser structure generated in order to reduce the surface energy due to the increase of the surface area of the system and the increase of the surface energy. Generalmente, due to the bridging effect of macromolecular surfactants or water-soluble polymers, the particles are connected in series to form a clump with a loose structure like cotton wool. In this structure, the distances between the ions are much larger than in aggregates or agglomerates.
Agglomeration and dispersion of particles in liquids
The wettability of particle surface is of great significance to powder dispersion and is the theoretical basis for powder dispersion, solid-liquid separation, surface modification and granulation. The wetting of solid particles by liquids is mainly based on the wettability of the particle surface. The wettability of a solid surface is determined by its chemical composition and microstructure. The higher the free energy of a solid surface, the easier it is to be wetted by a liquid; y viceversa. Por lo tanto, seeking and preparing solid surfaces with high surface free energy has become a prerequisite for the preparation of superhydrophilic and superhydrophobic surfaces.
Agglomeration and dispersion of particles in liquids
The wettability of particle surface is of great significance to powder dispersion and is the theoretical basis for powder dispersion, solid-liquid separation, surface modification and granulation. The wetting of solid particles by liquids is mainly based on the wettability of the particle surface. The wettability of a solid surface is determined by its chemical composition and microstructure. The higher the free energy of a solid surface, the easier it is to be wetted by a liquid; y viceversa. Por lo tanto, seeking and preparing solid surfaces with high surface free energy has become a prerequisite for the preparation of superhydrophilic and superhydrophobic surfaces.
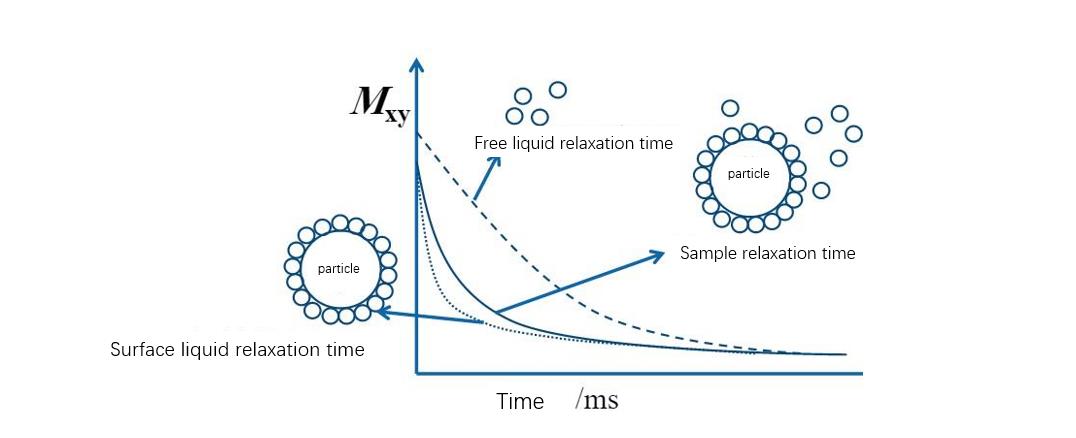
Low-field nuclear magnetic technology to study the principle of particle agglomeration state:
For wet particle systems, a layer of liquid-phase molecules is attached to the particle surface, and these liquid-phase molecules are restricted in motion due to adsorption on the surface of the inorganic phase. Sin embargo, the motion of liquid molecules that are not in contact with particles is free, and the relaxation time of liquid molecules is closely related to the motion state it is in. The NMR relaxation time of liquid molecules in a free state is longer than that in a bound state. The relaxation time of the liquid phase molecules in the state is much longer, and the system with better particle dispersion has relatively more solvent adsorption and shorter relaxation time. Por lo tanto, the low-field nuclear magnetic resonance technique can be used to measure the relaxation time of the suspension system and calculate the wetting specific surface area (available adsorption surface area) of the particles, which can then be used to study the agglomeration state, dispersion stability, affinity of the particles properties and wettability.
 mohoso
mohoso