Fluorine content Measurement
Accurate measurement of fluorine content is essential for understanding its distribution, effects, and applications in various fields. It enables scientists, researchers, and industries to make informed decisions, ensure regulatory compliance, and promote health and environmental safety.
Measuring fluorine content refers to determining the amount or concentration of fluorine present in a sample. Fluorine is a chemical element with the symbol F and atomic number 9. It is highly reactive and occurs naturally in various minerals and compounds.
Fluorine content Measurement Methods
The measurement of fluorine content can be performed using various techniques depending on the specific sample and the level of accuracy required. Here are a few common methods used for fluorine content measurement:
Potentiometric Titration: In this method, a known volume of a solution containing a strong base, such as sodium hydroxide (NaOH), is added to the sample solution. The excess base reacts with the fluorine present in the sample, forming a known amount of fluoride ions. The remaining unreacted base is then titrated with an acid using a pH electrode until the endpoint is reached. The amount of acid used is proportional to the fluorine content in the sample.
Ion-Selective Electrode (ISE) Method: This method uses an ion-selective electrode specifically designed for fluorine ions. The sample is typically dissolved in water, and the electrode measures the potential difference generated by the fluoride ions in the solution. The concentration of fluorine can be determined by comparing the electrode response to a calibration curve.
Ion Chromatography (IC): IC is a widely used technique for the analysis of various ions, including fluoride. In this method, the sample is injected into an ion chromatograph equipped with a fluorine-specific column. The fluoride ions are separated from other ions present in the sample, and their concentration is determined by comparing the peak area or height to a calibration curve.
Gravimetric Method: This method involves the precipitation of fluoride ions as calcium fluoride (CaF2) or another insoluble fluoride salt. The sample is dissolved in water, and a known excess of calcium chloride (CaCl2) or another soluble calcium salt is added. The resulting precipitate is filtered, washed, dried, and weighed. The weight of the precipitate is used to calculate the fluorine content.
Spectroscopic Methods: Fluorine can also be measured using spectroscopic techniques such as X-ray fluorescence (XRF) or inductively coupled plasma optical emission spectroscopy (ICP-OES). These methods are often employed for analyzing solid samples or complex matrices where other methods may not be suitable.
NMR Methods: Low field NMR spectroscopy can be used to measure the fluorine content in samples. While high field NMR spectrometers (e.g., operating at 300 MHz or higher) are typically used for routine NMR measurements, low field NMR instruments (e.g., operating at frequencies below 100 MHz) have advantages such as lower cost, portability, and ease of use.
It’s important to note that the choice of method depends on factors such as the nature of the sample, required accuracy, cost, and availability of equipment. Additionally, it’s advisable to consult appropriate references, analytical standards, and experienced analysts to ensure accurate measurement of fluorine content.
Fluorine Content of Fluoride Toothpaste
Fluoride toothpaste refers to a type of toothpaste that contains fluoride as an active ingredient. Fluoride is a naturally occurring mineral that has been shown to be highly effective in preventing tooth decay and promoting dental health. Using fluoride toothpaste as part of a regular oral hygiene routine, along with proper brushing and flossing techniques, can significantly contribute to maintaining good dental health, preventing tooth decay, and promoting strong teeth. It is advisable to follow the recommendations of dental professionals and consult with a dentist for personalized oral care advice.
Fluorine Content Measurement by NMR
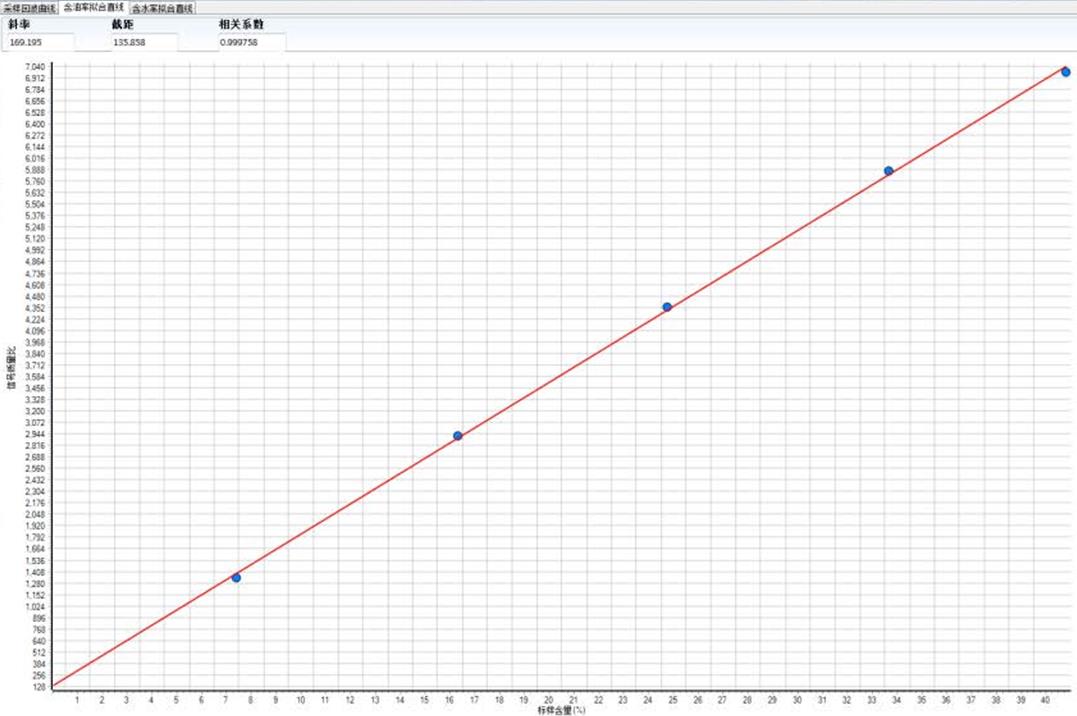
The NMR method is based on the linear relationship between the content of fluorine nuclei in the sample and the intensity of the NMR signal. The NMR signal amplitude is proportional to the mass of soluble fluorine, and through this linear relationship, the fluorine content of the sample to be tested can be quickly detected.
Low field NMR spectroscopy can be particularly useful in applications where portability and cost-effectiveness are prioritized, such as on-site analysis, quality control in industrial processes, or field studies.
 NIUMAG
NIUMAG