Introduction
Coal water according to the different can be divided into the adsorption state of occurrence state of water (adsorbed water (mainly small hole), capillary bound water, capillary water (within Yu Zhongkong) and free water (free water) (Yu Dakong and fissure);And the gas in the coal (here mainly refers to methane) also according to the different occurrence can be divided into three types: adsorption state (adsorbed methane), hole bound state (porous medium confined methane) and free (bulk methane).Since there is such a difference in the occurrence space, gas and water can be accurately identified by using the advantages of low field nuclear magnetism in pore size characterization.
This problem is often encountered in coalbed methane development, where fluid in the formation or from outside may enter the coal reservoir during well shut-in and workover, and other stimulation measures such as hydraulic fracturing enter the coal reservoir. It is not clear what effect this fluid has on the desorption and migration of the coal gas.
Sample Preparation
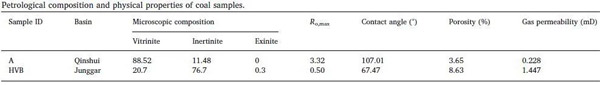
Anthracite (hereinafter referred to as high-rank coal) from qinshui basin and high-volatile bituminous coal (hereinafter referred to as low-rank coal) rock core nmr device from junggar basin were selected for the experiment. The sample information is shown in the following table. The wetting Angle test shows that the high rank coal is hydrophobic, while the low rank coal is obviously hydrophilic. At the same time, the porosity and permeability of low rank coal is much better than that of high rank coal.
Principle
MesoMR23-060H-I produced by Niumag was used in this low-field NMR experiment, as shown in the figure below.

First, let’s quickly review the basics of low field nuclear magnetism. This technique is to study the physical process of hydrogen core of reservoir fluid absorbing a certain frequency of rf pulse under the action of external magnetic field to restore the equilibrium state after nuclear magnetic resonance, and analyze the fluid characteristics in the pores of reservoir rock by measuring the transverse relaxation time T2 of hydrogen core. The T2 distribution characteristics of reservoir fluid are affected by three relaxation mechanisms: surface relaxation, body relaxation and diffusion relaxation, which can be expressed as:
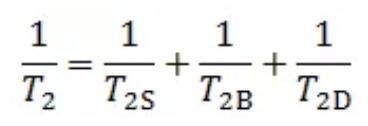
T2S —surface relaxation,ms;
T2B —body relaxation,ms;
T2D —diffusion relaxation,ms;
If the uniform magnetic field is used in the experiment, the diffusion relaxation can be ignored. Moreover, since the interaction between the fluid in the rock and the pore surface is very strong, the surface relaxation time T2S of the reservoir fluid is far less than the body relaxation time T2B, so 1/T2B can be ignored. The above formula can be further expressed as:
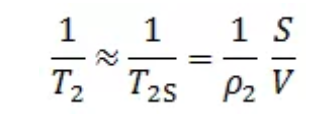
Where, S is the surface area of the pores; V is the pore volume.
Further expressed as:
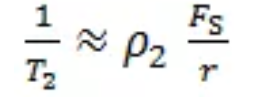
Therefore, the rock relaxation time T2 is proportional to the pore radius and inversely proportional to the relaxation rate. The shorter the relaxation time, the smaller the pore radius of the rock.
Experiment
First, Stage1 (figure 2) conducted nuclear magnetic measurement for dried coal samples, with the purpose of ensuring that the adsorption curve measured by nuclear magnetic method is highly consistent with the results of isothermal adsorption method.
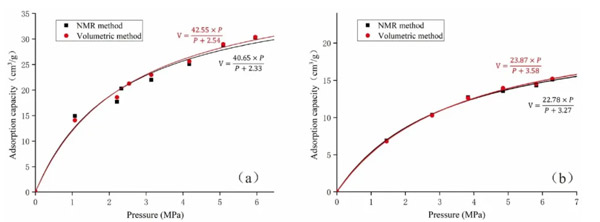
It can be seen from figure 1 that for methane adsorption of dried samples, the results of nuclear magnetic field and traditional adsorption method are completely acceptable within the error range.
You might be wondering here, since traditional isothermal adsorption experiments can also obtain adsorption curves, why do we have to use nuclear magnetism? This is because the traditional method can only give the total amount of water and adsorbed gas in coal, but cannot obtain the amount of different pore Spaces and different occurrence states.
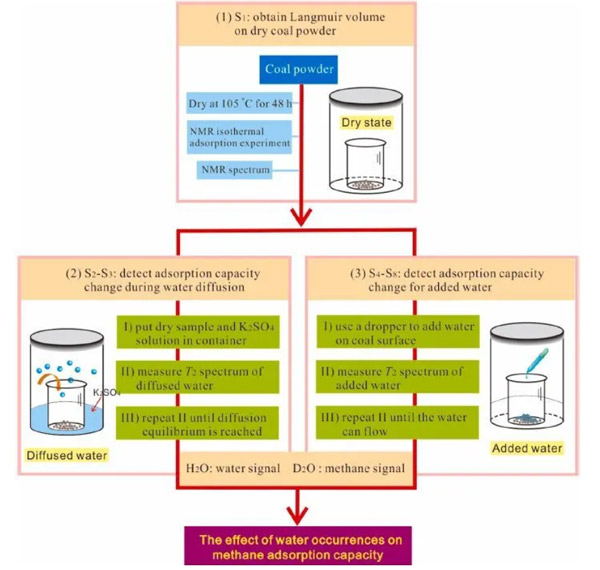
After the nuclear magnetic calibration, two sets of experiments were carried out, stage2-3 (water free diffusion in coal) and stage4-8 (observation process after water enters), and water was added to the surface of coal particles. This is because we suspect that water that diffuses freely into the coal matrix will eventually tend to adhere to the surface of the tiny pores in the form of adsorption, while the water that is added to the coal matrix will first exist in the free state in the macropores and may gradually enter the smaller pores over time.
There are two important points to note in the experiment: first, to accelerate the relaxation time of water, K2SO4 solution was used instead of pure water; But in order to distinguish the signals of water and gas, when we care about the spectral peak of water, we choose ordinary water H2O, and when we care about the spectral peak of gas, we choose heavy water D2O to repeat the experiment.
 NIUMAG
NIUMAG