Низкое полевое исследование ЯМР по процессу отека коллоида
The swelling process of the hydrophilic colloid is the phenomenon that the volume of the polymer increases after absorbing the liquid. The molecular structure of colloidal compounds contains many hydrophilic groups, which can interact with water molecules. After hydration, a similar molecular state is dispersed in water to form a hydrophilic colloid solution. Such as animal glue, aqueous solutions of enzymes and other biochemical preparations containing protein, natural polysaccharides, mucilage and gums, и т. д., are all colloidal solutions formed when they meet water. The vast majority of hydrophilic colloids are macro-molecular compounds, so the hydrophilic colloid solution is also called macro-molecular aqueous solution. Whether swelling occurs or not depends on the properties of the polymer and liquid. Linear polymers swell first and then dissolve, while bulk polymers only swell but do not dissolve. Например, gelatin swells in water but not in organic solvents; rubber swells in benzene but not in water. Some polymers form sols after swelling. Например, gelatin in water and rubber in benzene form a sol when heated.
Sol is also called colloidal solution. A dispersion system formed by dispersing particles of dispersoid in a medium. According to the relationship with the liquid dispersion medium, it can be divided into two categories: lyophilic sol and lyophobic sol. Compared to the undispersed material, the particles of the dispersed phase are very small and the total area is very large, which is a characteristic of sols.
The swelling process and the peptization process are actually the redispersion process of the colloidal particles. Colloidal particles themselves have certain stability, such as charge repulsion, the existence of a hydration layer, и т. д.. When these conditions disappear, the colloidal particles will agglomerate, so heating, adding electrolytes, adding oppositely charged colloids, и т. д.. is nothing more than removing the charge, removing the hydration layer (or solvent layer), and making the colloids agglomerate together.
After the colloid is agglomerated, it may be further dehydrated and chemically reacted to form chemical bonds, so that it will not dissolve and disperse again. Однако, it is also possible to recombine water or solvent. В это время, the agglomerated colloidal particles will increase in volume (due to the addition of solvent between the particles), swell, or even completely disperse, solvate, and peptize.
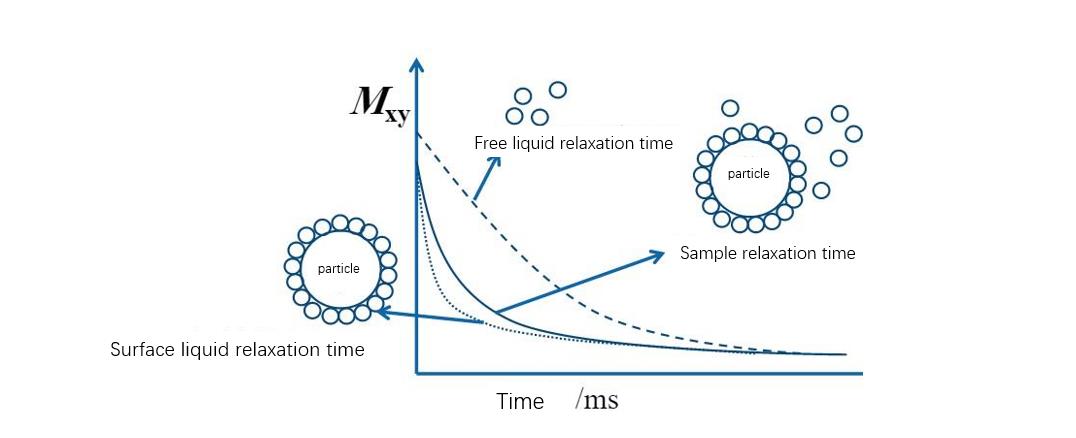
Low-field NMR technology to study the swelling process of colloids:
Ядерный магнитный резонанс низкого поля (Низкопольное ЯМР) has great potential to study water transport and micro-structure of water mobility-based polymer networks. Unlike high-resolution NMR, low-field NMR (Низкопольное ЯМР) is mainly used to indicate structural heterogeneity and molecular mobility of interactions by measuring relaxation times. Studies have shown that low-field nuclear magnetic resonance (Низкопольное ЯМР) это пост, non-destructive method for determining the distribution of water components. This method can quickly evaluate the agglomeration and dispersion state of the particle stock solution, and can be used for the study of colloid swelling process.
 заплесневелый
заплесневелый