Introduction
Shale has low permeability, low porosity, high clay content, and high organic matter content. The lacustrine shale has strong heterogeneity and variable organic matter content. Stratification is a common feature of shale and the main reason for shale heterogeneity. Shale consists of two parts: (a) a clay-organic matrix containing clay minerals and organic matter; and (b) inclusions such as quartz, feldspar and pyrite. Shale pores are mainly composed of clay-related pores and intergranular pores. The physical structure of shale is not conducive to the production of shale oil. In addition, due to the absorption and adsorption of hydrocarbons, the highly variable organic content is also not conducive to the production of shale oil.
Due to clay swelling, low injection, and low impact range, it is difficult to apply water flooding in shale reservoirs, and effective measures for enhanced recovery are urgently needed.
Laboratory and field tests have proven that carbon dioxide injection is one of the most effective ways to increase recovery Industry NMR. Carbon dioxide can reduce interfacial tension and viscosity of crude oil; carbonic acid can dissolve calcium carbonate and increase permeability; mixed phase injection pressure of carbon dioxide is lower than nitrogen and methane. In addition, carbon dioxide also has a competitive adsorption effect, and the hydrocarbons adsorbed and absorbed by organic matter can be replaced by carbon dioxide.
The adsorption process of molecules in porous media includes micropores and surface diffusion and surface adsorption. The more molecules are adsorbed on the pores, the more difficult it is for other molecules to be adsorbed. Therefore, the adsorption rate is negatively correlated with the concentration of the adsorbed phase and positively correlated with the concentration of the free phase.
For shale, oil absorption by organic matter also needs to be considered. In fact, it is difficult to distinguish between micropores and surface diffusion in organic matter and surface adsorption and diffusion in oil. The total adsorption absorption rate is proposed to describe the following processes: micropores and surface diffusion, surface adsorption, and absorption in organic matter. Molecular diffusion is also an important process of low-permeability miscible carbon dioxide flooding, which can be expressed by the Maxwell-Stefan (MS) equation.
Nuclear magnetic resonance provides a very good solution for the measurement of the dynamic changes of adsorption and absorption in the carbon dioxide miscible flooding process. NMR is measured by testing the hydrogen signal nucleus in the fluid. The amplitude of the NMR signal is proportional to the number of hydrogen nuclei. The lateral relaxation time T2 is proportional to the pore size. In the NMR T2 distribution, the water response in mud shale is divided into three parts: clay-bound water, bound water, and free water.
The typical T2cutoff of clay-bound water is about 3 ms. Unconventional reservoirs (such as shale reservoirs) contain hydrocarbons in the form of oil and asphalt, which are formed in the kerogen matrix during maturation and have relaxation times similar to clay-bound water.
This paper uses NMR technology to perform carbon dioxide miscible flooding experiments on oil-bearing shale and sandstone under high pressure. According to the NMR T2 spectrum distribution curve, the oil in shale is divided into two parts: fixed oil and free oil. Through the change of NMR T2 spectrum distribution curve during the miscible flooding of CO2, the recovery factors of oil in different occurrence states (fixed oil and free oil) were obtained. In addition, the effects of shale heterogeneity, competitive adsorption and absorption of carbon dioxide-oil mixtures on shale carbon dioxide miscible flooding were studied by numerical simulation.
Experiment
Materials
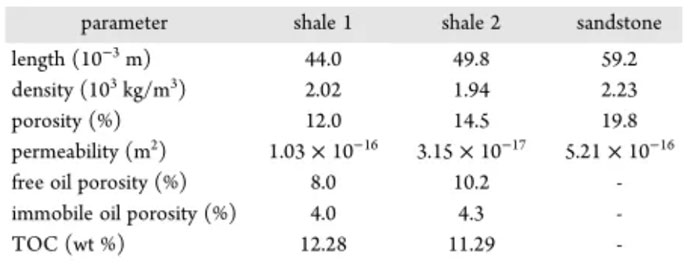
The experimental samples were taken from shale formations in the Ordos Basin. The basic parameters of the samples are shown in Table 1.
Table 1 Physical and geochemical characteristics of the samples
The mineral composition of the sample measured by X-ray diffraction (XRD) is shown in Table 2. The mineral composition of shale is more complex than that of sandstone. The main mineral components of sandstone are quartz and plagioclase, while shale contains clay, quartz and other minerals.
Table 2 Mineral composition of the samples
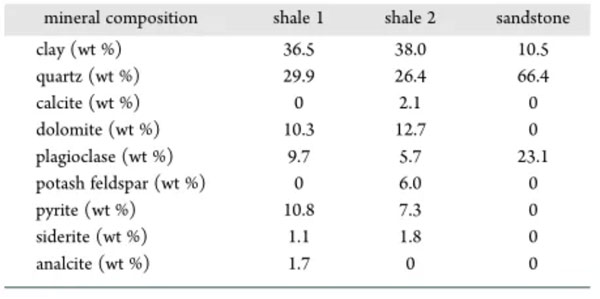
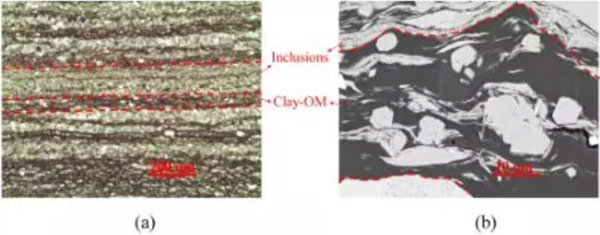
Figure 1 shows the stratification of shale samples at different magnifications. The shale sample consists of clay-organic and encapsulating phases. On the macro scale, the encapsulation phase (leucorrhea) is embedded in the clay-organic phase; on the micro scale, some white particles are discretely embedded in the clay-organic phase. Because the white particles are relatively isolated in the clay-organic phase, their influence on fluid migration is small.
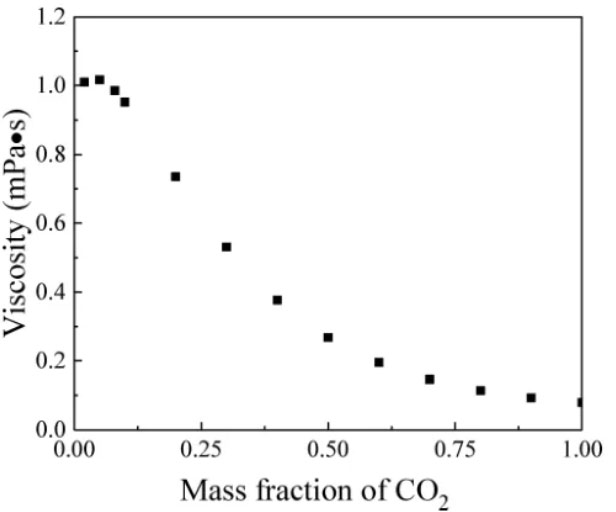
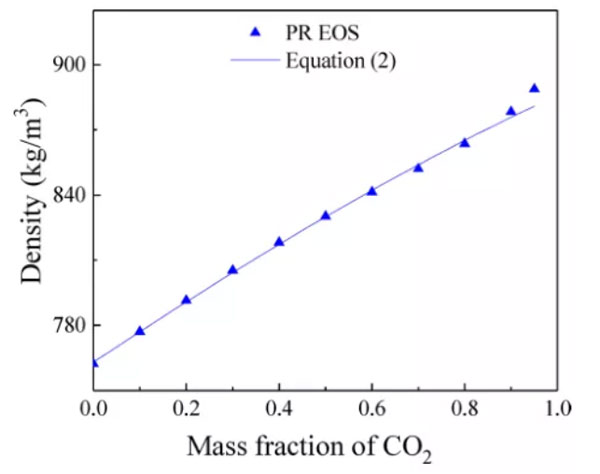
We use n-dodecane as the oil phase. The viscosity and density of the carbon dioxide-n-dodecane mixture during the mixed-phase injection process vary with the carbon dioxide mass fraction, as shown in Figures 2 and
CO2 Miscible Flooding Experiment Based on Nuclear Magnetic Resonance
CO2 miscible oil displacement experimental device based on nuclear magnetic resonance is shown in Fig. 4。
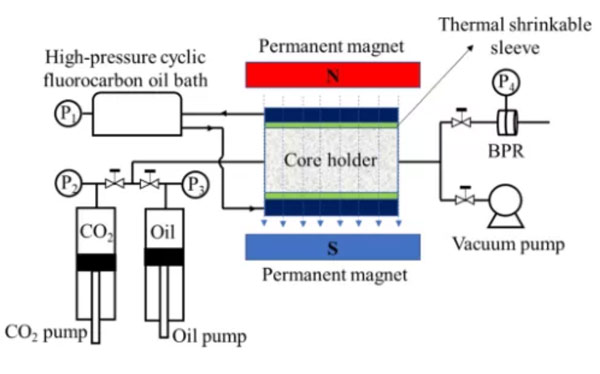
The experimental steps are as follows:
(1) Wash the sample with dichloromethane for 15 days and dry for 24 hours before the experiment.
(2) Put the sample into the core holder and keep it at least 5 hours under the set confining pressure and temperature to reach the stress equilibrium.
(3) The core holder is evacuated by a vacuum pump for at least 24 hours until the pressure reaches 0.002% of atmospheric pressure.
(4) Inject the oil into the core holder at a pressure of 15.0 MPa until the T2 spectrum remains stable.
(5) The carbon dioxide is injected into the core holder through a carbon dioxide pump. The injection pressure of carbon dioxide is 16 MPa, the outlet pressure is 15 MPa, the displacement pressure difference is 1 MPa, and the T2 distribution is measured by a nuclear magnetic resonance instrument in real time.
 NIUMAG
NIUMAG