Temperature changes during pore water freezing
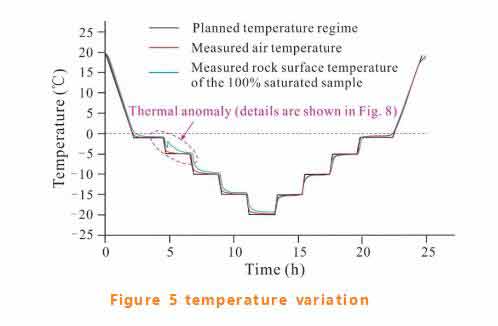
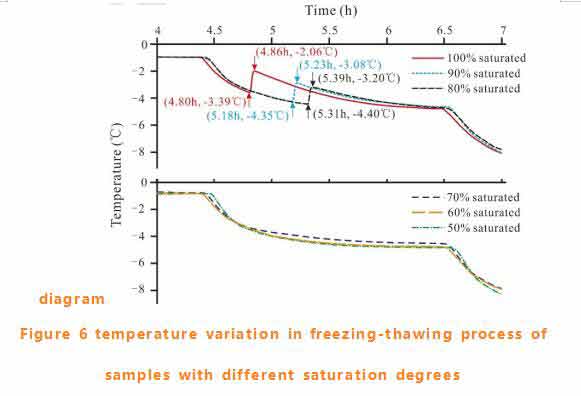
The air temperature in the tank and the rock surface temperature were tested, and the results were consistent with the set temperature range (see figure 5).The fully saturated coal sample has thermal anomalies within the range of -1℃ to -5℃. The enlarged figure in this area is shown in figure 6.
Sudden increases in rock surface temperature represent latent heat release, which is a clear sign of pore water freezing. As shown in figure 6, the rock surface temperature of the fully saturated sample increased rapidly from -3.39℃ to -2.06℃.The range of temperature rise decreases with the decrease of the saturation degree of the sample. When the saturation is less than 70%, the temperature rise cannot be detected.
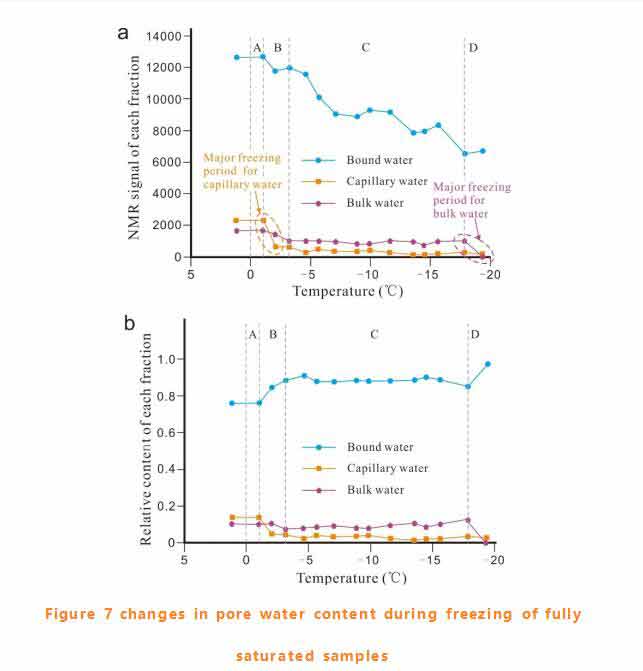
Pore water freezing mode for fully saturated samples
We summarized the content changes of different components of pore water during the freezing process of completely saturated sandstone. The absolute content changes of each component were shown in figure 9a and the relative content changes in figure 9b.The whole pore water freezing process can be roughly divided into four sections :a) supercooled section (0~-1℃);B) fast freezing section (-1~-3℃);C) stable freezing section (-3~-18℃);D) freezing stop section (-18~-20℃).
The content of components in supercooled section remains unchanged. With the beginning of freezing, the content of bound water and capillary water in the fast freezing section decreased significantly, while the mass fraction of bound water and capillary water increased and decreased respectively. Free water decreases slightly and its mass fraction remains almost unchanged. Free water exists in macropores, capillary water in smaller pores (mesoporous), bound water adsorbs on the surface of mineral particles or condenses in smaller pores (micropores).The changes of the components of pore water in the fast freezing section indicated that the pore was frozen before the macropore.
In the stable frozen section, the bound water content fluctuates and the mass fraction is almost unchanged. The capillary water content decreases gradually and approaches 0 when it exceeds -15℃.Free water decreases slightly. Film water adsorbs on the surface of mineral particles and remains unfrozen at sub-zero temperature under the action of adsorption. As pore water crystallizes and ice grows, adsorbed water increases, so more bound water remains on the ice surface. Free water increases mainly at the end of freezing, perhaps due to the presence of salt in pore water, and because the sample is saturated with tap water it is likely to contain soluble salt.
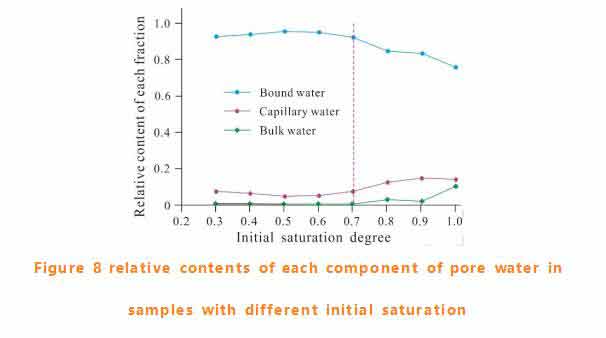
Effect of initial moisture content on freezing process of sandstone
The variation of unfrozen water content and latent heat release during freezing are closely related to initial saturation. The freezing process can be predicted by the composition of pore water before freezing. The saturation of 70% is considered as the critical value of freezing mode change. It is speculated that the transition is due to the change of free water at this point. Since undercooling is required before nucleation, free water may play an important role in the crystallization of pore water by forming an undercooling base. In the known critical saturation of freezing damage in porous rock, 70% is a frequently mentioned value.NMR Analyzer This paper also provides a possible explanation for the rationality of this critical value.
Conclusion
In this paper, the freezing process of pore water in sandstone samples with different initial water saturation was tested by low-field nuclear magnetic resonance method. The conclusions are as follows:
1) Completely saturated sandstone samples contain bound water, capillary water and free water. Bound water is the main, capillary water is the second, free water is the least. At -20℃, there is still a large amount of bound water has not been frozen.
2) When the saturation of > is 70%, the phenomenon of supercooling of pore water is obvious. The variation of unfrozen water content in freezing and thawing process has hysteresis phenomenon. The lower saturation is, the smaller hysteresis loop is.
3) Due to the release of latent heat, there is a sudden increase in the rock temperature. When saturation < 70%, the burst disappears.
4) The freezing process can be divided into four sections: supercooled section, rapid freezing section, stable freezing section and freezing termination section. In the fast freezing section, the pore is frozen before the macropore.
5) Initial saturation influences freezing process by changing pore water composition before freezing.
 NIUMAG
NIUMAG